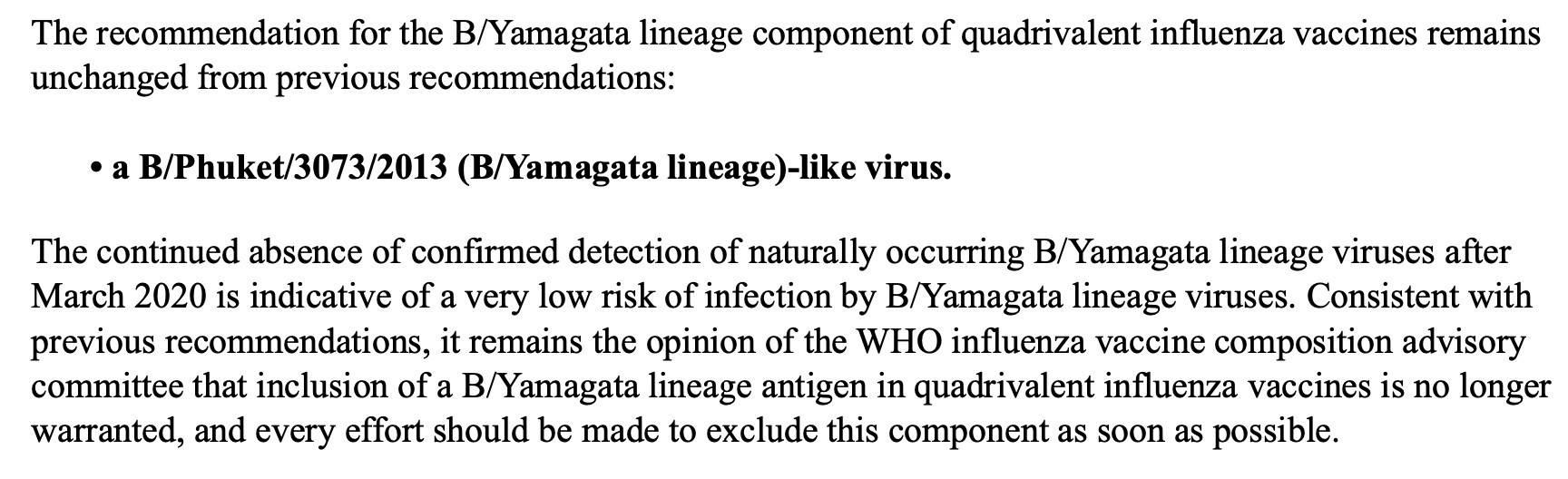
5/ Recommended composition of influenza virus vaccines for use in the 2025 southern hemisphere influenza season 💉 WHO recommends trivalent vaccines for use in 2025 southern hemisphere influenza season 🧪 Contains a H1N1pdm09-like virus, a H3N2-like virus & a B/Victoria lineage-like virus /end

4/ Technical briefing content 🌍 Summary of seasonal influenza activity in different global transmission zones 🔬 Genetic and antigenic characteristics of recent seasonal influenza viruses, human serology & antiviral susceptibility 💉 The recommendation for composition of influenza virus vaccines
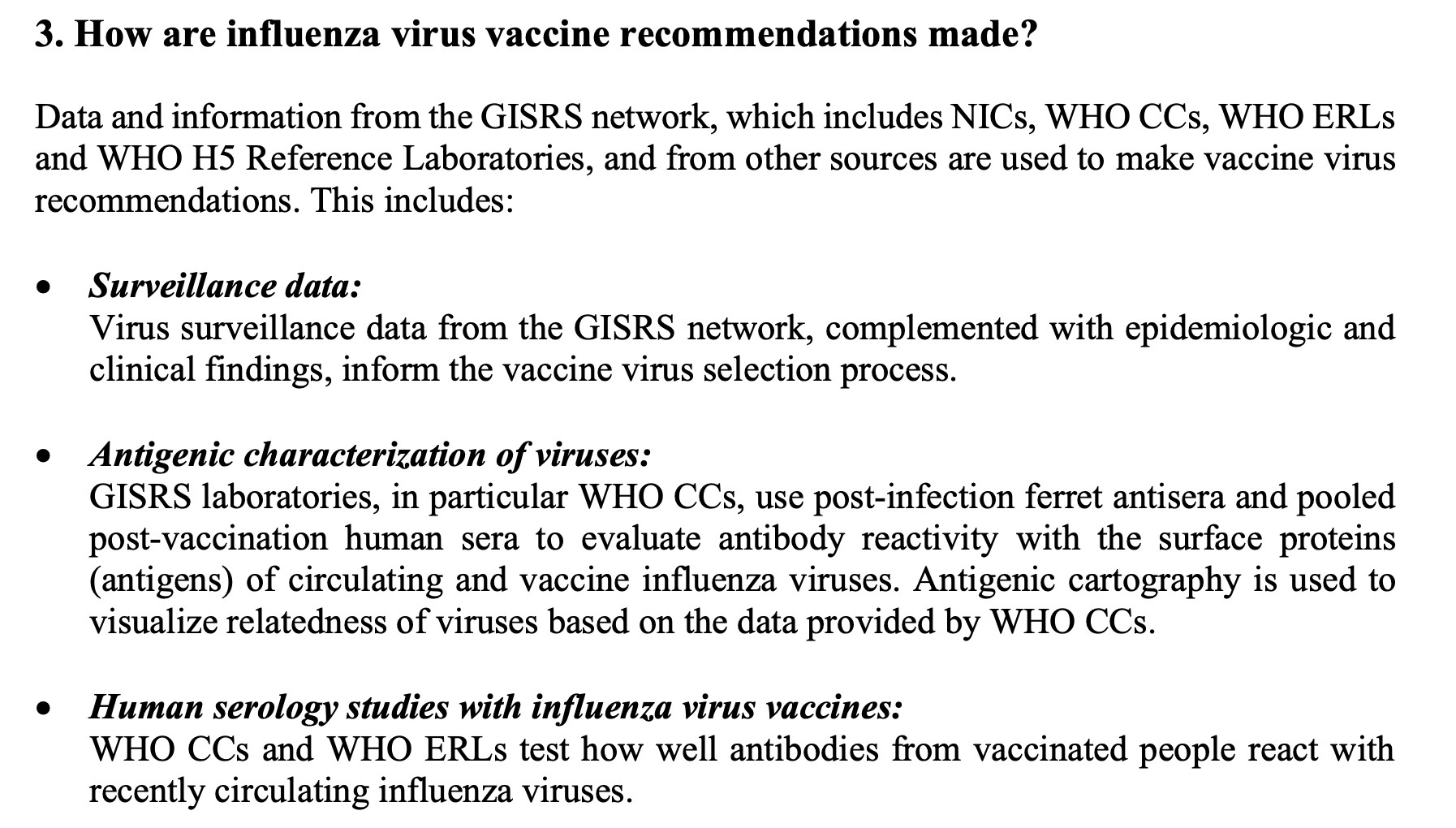
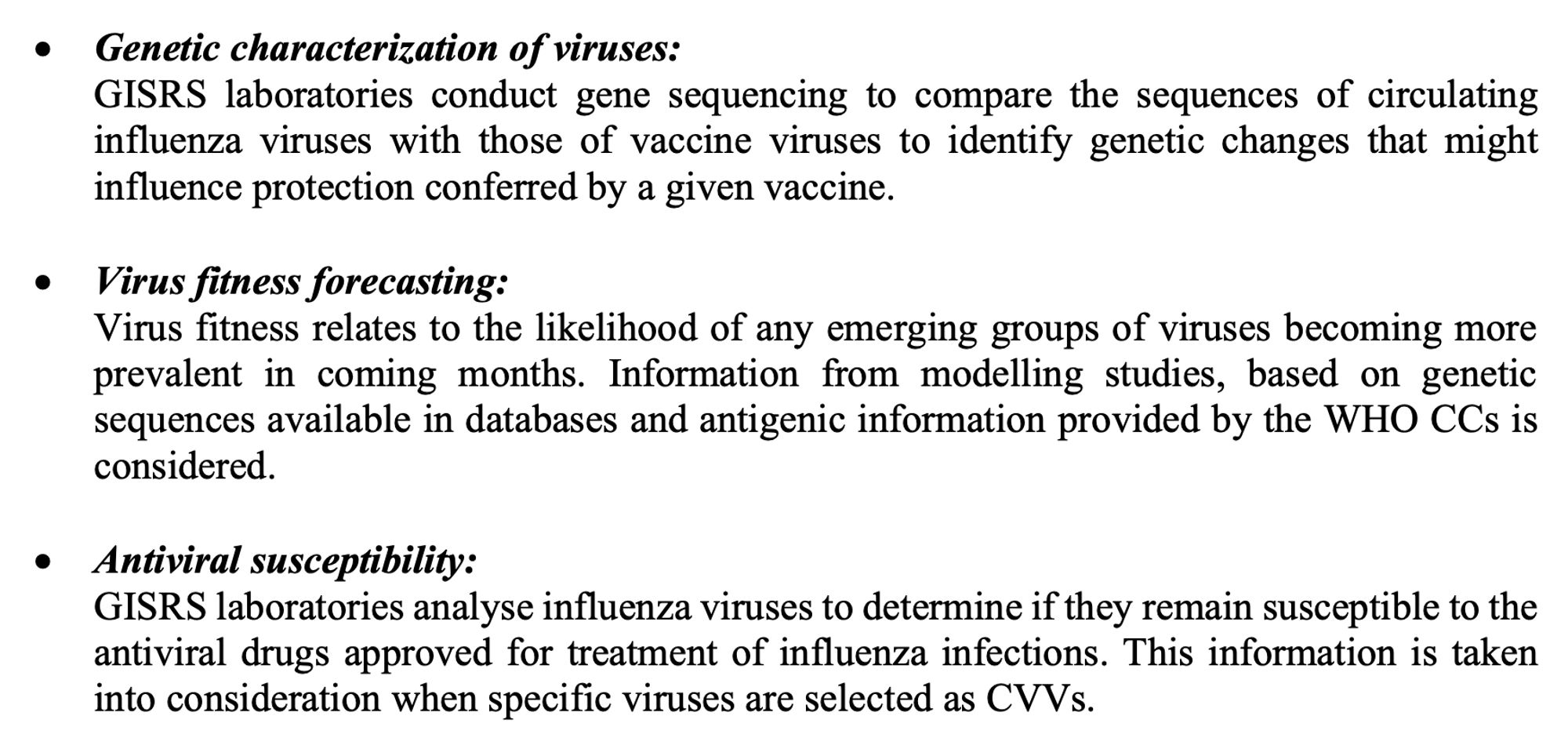
3/ How are influenza virus vaccine recommendations made? 📊 Uses data from WHO Global Influenza Surveillance & Response System (GISRS) Data types mentioned in FAQ doc include: ▶️ Surveillance data ▶️ Antigenic & genetic characterisation of viruses ▶️ Human serology studies ▶️ Vaccine effectiveness


2/ Purpose of WHO recommendations on influenza virus vaccine composition? ▶️ Periodic update of influenza vaccines is necessary due to constant evolving nature of influenza viruses 🗓️ WHO holds technical consultations twice annually (Feb & Sep), once for southern & once for northern hemispheres

1/ How does WHO make seasonal influenza vaccine composition recommendations❓ 🧪 Recommendations announced (on 27 Sep 2024) for 2025 southern hemisphere influenza season 🔗 News release: www.who.int/news/item/27...www.who.int/publications...cdn.who.int/media/docs/d...

Publications of the World Health Organization
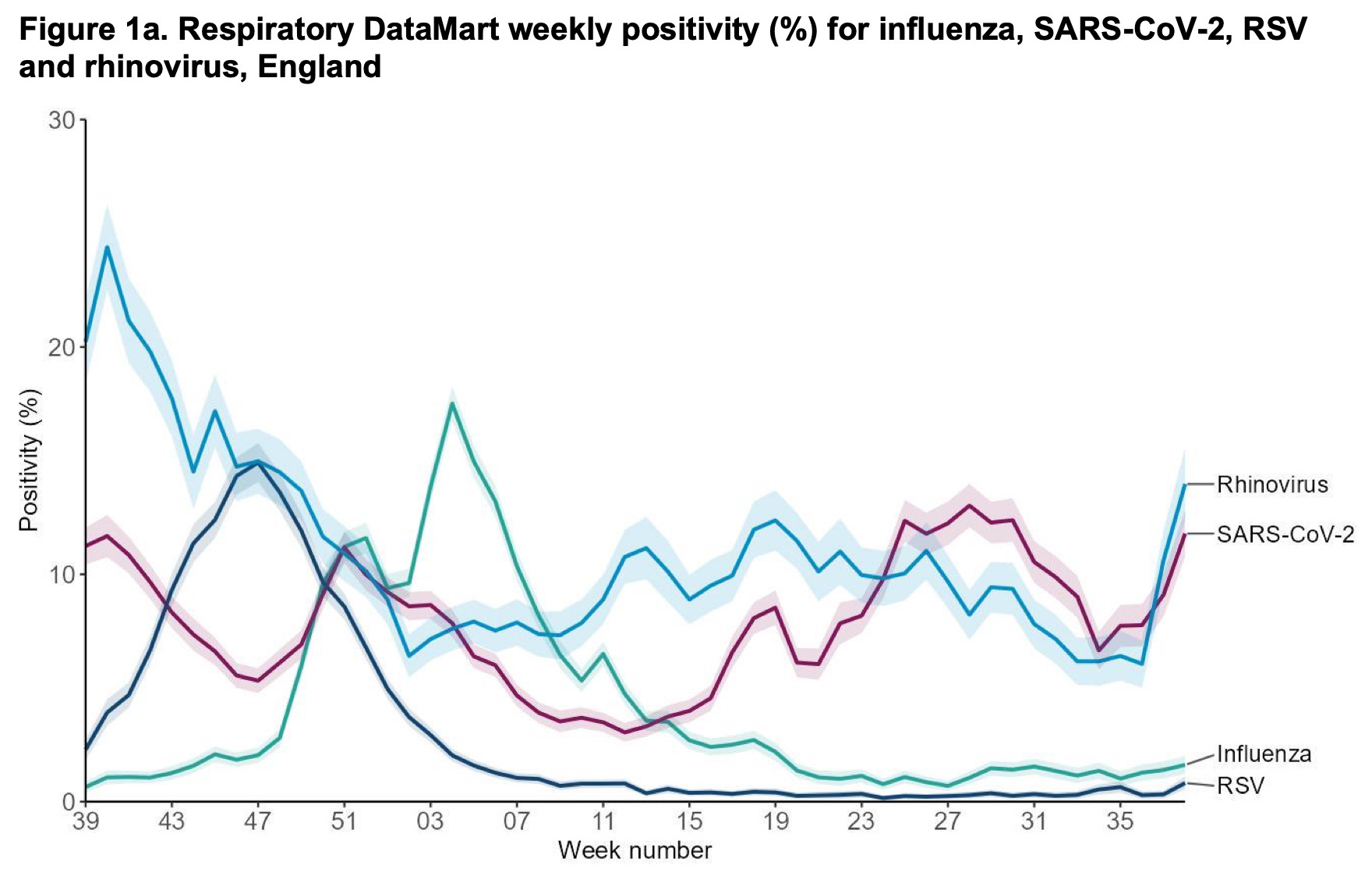
Informative discussion - see both post and replies on what possible cluster of human bird-flu infections in Missouri says about H5N1 avian influenza transmission potential. 🧪 #EpiSky
This is bad from a surveillance point of view. In the event that there were several human infections with a new H5N1 flu in the same cluster that would tip it so a wider outbreak was more likely than not in my estimation. But we don't know because it wasn't tested for.
More from Missouri: #CDC reports that Missouri has found 4 more health workers who developed mild illness after caring for the confirmed #H5N1 #birdflu case hospitalized in August. None were tested while ill. www.statnews.com/2024/09/27/b...
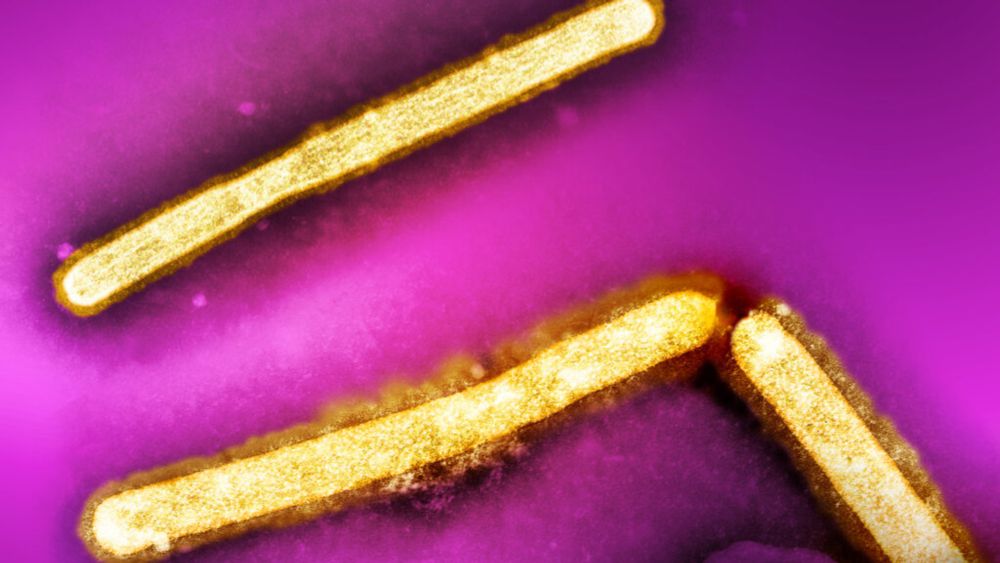
The CDC said an investigation into a human H5N1 bird flu infection in Missouri has found four additional health care workers who fell ill after caring for the patient.
4/ Research gaps ❓ Should the West vaccinate poultry for H5N1? ❓ Can H5N1 be eliminated in US dairy cattle? ❓ Prospects for the Future - severity of a future H5N1 pandemic remains unclear /end
3/ How could H5N1 become a pandemic? ▶️ Examines how changes in the ecology & molecular evolution of H5N1 in wild & domestic birds increases opportunities for spillover to mammals. ▶️ Discusses the chance of different evolutionary pathways that could turn H5N1 into a pandemic virus